| Product | Discovery | Pre-clinical Studies | Phase I Studies | Phase II Studies | Phase III Studies | BLA / MAA | Market |
|---|---|---|---|---|---|---|---|
| TMB-365 | Discovery
|
Pre-clinical Studies
|
Phase I Studies
|
Phase II Studies
|
Phase III Studies
|
BLA / MAA
|
Market
|
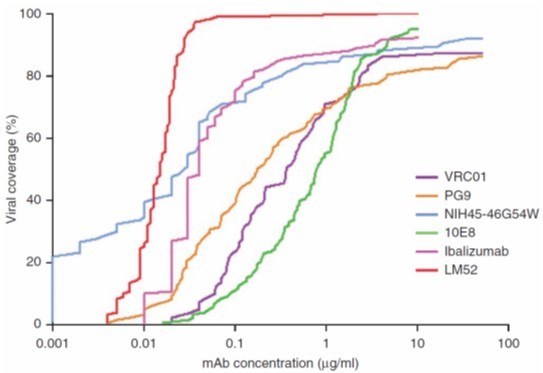
TMB-365 is the second-generation TMB-355 monoclonal antibody used for HIV treatment and prevention.
It exhibits better drug resistance and efficacy compared to TMB-355. Developed by a team led by Dr. David Ho, TaiMed holds exclusive global rights for its development and marketing. Product Function Description
For HIV
TMB-365 Injection
TMB-365 is a long-acting, broadly-blocking antibody improved from the commercialized ibalizumab. It is an effective post-attachment entry inhibitor that preventing HIV-1 from entering the CD4+ cells by attaching to the CD4 receptors on their surface. Much like ibalizumab, TMB-365 operates through a unique CD4-driven mechanism of action. The feature allows the long-acting TMB-365 to be paired with other antiretroviral agents for effective treatment of HIV. In a Phase 1 clinical study, intravenous infusion of TMB-365 demonstrated good tolerability, safety, long-acting properties, and effectiveness against HIV infections when administered to HIV patients.
Phase 1 clinical trial
A phase-1 clinical trial was completed in 2021. Based on the study results, TMB-365 demonstrated a superior antiviral activity without any safety concerns. The pharmacokinetic (PK) results were better than expected and showed the potential for bi-monthly or quarterly dosing. With the promising data from the phase-1 study, TaiMed has submitted an IND application to US FDA for the combination of TMB-365 and TMB-380.
-
2019-10-012021
Completion of a phase-1 clinical trial. -
2019-10-012022
Initiation of a phase-1b/2a clinical trial for the combination of TMB-365 and TMB-380.